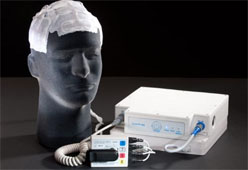
FDA Approves System for Patients with Brain Tumors
Oncology company Novocure said Friday that the U.S. Food and Drug Administration (FDA) has approved the NovoTTF-100A System (NovoTTF). The system is used for the treatment of adult patients with glioblastoma multiforme (GBM) brain tumors, following tumor recurrence after receiving chemotherapy.
The portable, wearable device delivers an anti-mitotic, anti-cancer therapy as patients maintain their normal daily activities, says the company.
[ Also Read: Here are the 10 Symptoms of Oral Cancer ]“Our device provides patients and physicians with a novel, non-invasive alternative to chemotherapy that is safe and effective,” said Eilon Kirson, M.D., Ph.D., Novocure’s chief medical officer.
“The device allows for continuous treatment without the usual, debilitating side effects that chemotherapies inflict on recurrent GBM patients and indirectly on their families.”
According to the company, results from a 237-patient randomized pivotal trial demonstrated that compared to patients treated with chemotherapy, NovoTTF treated patients achieved comparable median overall survival times, had fewer side effects, and reported improved quality of life scores.
[ Also Read: New Pregnancy Prevention Pills for You ]Glioblastoma is said to be the most aggressive and most common form of primary brain tumor in the United States. The disease affects approximately 10,000 Americans each year.
The median overall survival time from initial diagnosis is 15 months with optimal therapy, and median survival from the time of tumor recurrence is only three to four months without additional effective treatment.
The disease is widely recognized as one of the most aggressive and deadly forms of cancer.
💛 Support Independent Journalism
If you find RMN News useful, please consider supporting us.